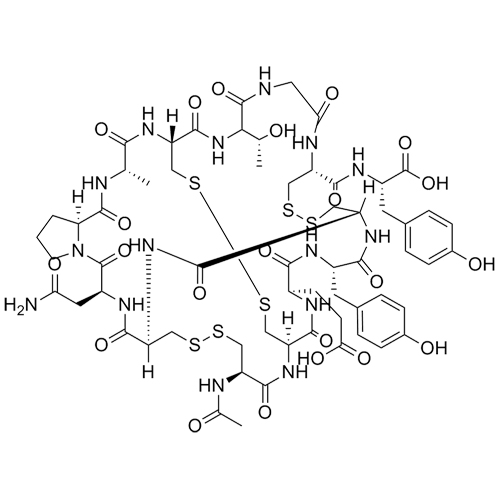
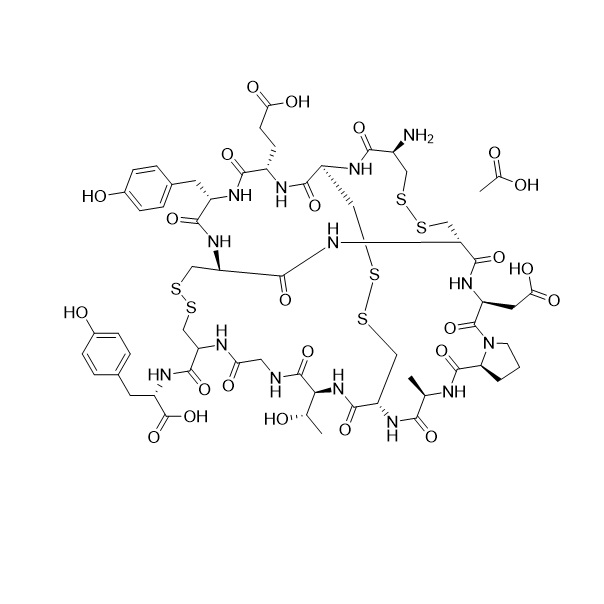
- Synonyms((3S,6R,9S,15R,20R,23S,26S,29R,32R,37R,40S,45aS)-32-acetamido-40-(2-amino-2-oxoethyl)-26-(2-carboxyethyl)-23-(4-hydroxybenzyl)-9-((R)-1-hydroxyethyl)-3-methyl-1,4,7,10,13,22,25,28,31,38,41,47-dodecaoxotetracontahydro-19H-37,20-(epiminomethano)-6,29-(methanodithiomethano)pyrrolo[2,1-s][1,2,27,28]t...
- Description
((3S,6R,9S,15R,20R,23S,26S,29R,32R,37R,40S,45aS)-32-acetamido-40-(2-amino-2-oxoethyl)-26-(2-carboxyethyl)-23-(4-hydroxybenzyl)-9-((R)-1-hydroxyethyl)-3-methyl-1,4,7,10,13,22,25,28,31,38,41,47-dodecaoxotetracontahydro-19H-37,20-(epiminomethano)-6,29-(methanodithiomethano)pyrrolo[2,1-s][1,2,27,28]tetrathia[5,8,11,14,17,20,23,32,35,38,41]undecaazacyclotritetracontine-15-carbonyl)-L-tyrosine acetate salt; : Ac-Cys-Cys-Glu-Tyr-Cys-Cys-Asn-Pro-AlaCys-Thr-Gly-Cys-Tyr-OH-(Cyclic (1-6), (2-10),(5-13)- tris(disulfide) Acetate salt.
Linaclotide N-Acetyl Acetate Salt is a fully characterized chemical compound used as a reference standard of API Linaclotide. The standard offered is compliant with regulatory guidelines. Linaclotide N-Acetyl Acetate Salt is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - NA
Related products
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...
Linaclotide
More options:
Enter Qty (# of units), Enter Qty (mg), Special...